Answer:
P = 2439.5 W = 2.439 KW
Step-by-step explanation:
First, we will find the mass of the water:
Mass = (Density)(Volume)
Mass = m = (1 kg/L)(10 L)
m = 10 kg
Now, we will find the energy required to heat the water between given temperature limits:
E = mCΔT
where,
E = energy = ?
C = specific heat capacity of water = 4182 J/kg.°C
ΔT = change in temperature = 95°C - 25°C = 70°C
Therefore,
E = (10 kg)(4182 J/kg.°C)(70°C)
E = 2.927 x 10⁶ J
Now, the power required will be:
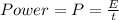
where,
t = time = (20 min)(60 s/1 min) = 1200 s
Therefore,
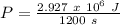
P = 2439.5 W = 2.439 KW