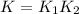The question is incomplete. The complete question is :
Hydrogen is manufactured on an industrial scale by this sequence of reactions:
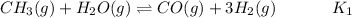
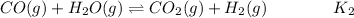
The net reaction is :
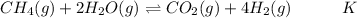
Write an equation that gives the overall equilibrium constant
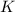 in terms of the equilibrium constants
in terms of the equilibrium constants
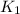 and
and
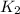 . If you need to include any physical constants, be sure you use their standard symbols, which you'll find in the ALEKS Calculator.
. If you need to include any physical constants, be sure you use their standard symbols, which you'll find in the ALEKS Calculator.
Solution :
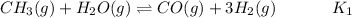
![$K_1 = ([CO][H_2]^3)/([CH_4][H_2O])$](https://img.qammunity.org/2022/formulas/chemistry/college/r74vzwm4viuwvrgj90pghyp66xr3g45346.png) ...............(1)
...............(1)
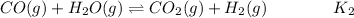
![$K_2 = ([CO_2][H_2])/([CO][H_2O])$](https://img.qammunity.org/2022/formulas/chemistry/college/7auynno6wd8s5q7uypygpbjtnjk51b3yu3.png) ...................(2)
...................(2)
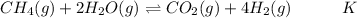
![$K=([CO_2][H_2]^4)/([CH_4][H_2O]^2)$](https://img.qammunity.org/2022/formulas/chemistry/college/e7125slr6nirvsbejdyfrvsiqat506s85w.png)
On multiplication of equation (1) and (2), we get
![$K_1 * K_2=([CO][H_2]^3)/([CH_4][H_2O]) * ([CO_2][H_2])/([CO][H_2O])$](https://img.qammunity.org/2022/formulas/chemistry/college/bjp4uttpg25banifv2jle9nqg8lu2btiyw.png)
![$K_1K_2=([CO_2][H_2]^4)/([CH_4][H_2O]^2)$](https://img.qammunity.org/2022/formulas/chemistry/college/88gzmr5k27mxn2u9pvhryc57p57arqqcl2.png) .................(4)
.................(4)
Comparing equation (3) and equation (4), we get