Answer:
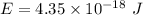
Step-by-step explanation:
Given that,
The distance between the electron and proton,

We need to find the change in electric potential energy. It can be calculated as follows :
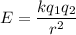
Where
k is the electrostatic constant
Change,
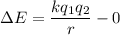
Put all the values.
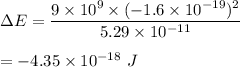
So, the required change in electric potential energy is
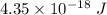 .
.