Answer: The empirical formula of one that contains 30.45% nitrogen is
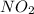 .
.
Step-by-step explanation:
Given: Mass of nitrogen = 30.45 g
Let us assume that the mass of given oxide is 100 grams.
As the atomic mass of nitrogen is 14.0067 g. So, moles of nitrogen will be calculated as follows.
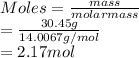
Also, mass of oxygen = (100 - 30.45) g = 69.55 g
Atomic mass of oxygen is 15.9994 g/mol. So, moles of oxygen will be as follows.
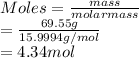
The ratio of both the atoms is as follows.
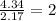
This means that gas has 2 moles of oxygen to 1 mole of nitrogen. Hence, the formula of oxide is
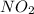 .
.
Thus, we can conclude that the empirical formula of one that contains 30.45% nitrogen is
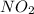 .
.