Answer: There are 0.024 moles of gas are present in the container.
Step-by-step explanation:
Given: Temperature =
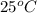 = (25 + 273) K = 298 K
= (25 + 273) K = 298 K
Pressure = 1.7 atm
Volume = 345 mL (1 mL = 0.001 L) = 0.345 L
Formula used is as follows.
PV = nRT
where,
P = pressure
V = volume
n = no. of moles
R = gas constant = 0.0821 L atm/mol K
T= temperature
Substitute the values into above formula as follows.
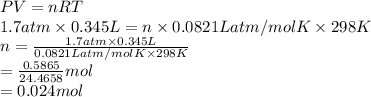
Thus, we can conclude that there are 0.024 moles of gas are present in the container.