Step-by-step explanation:
The given reaction is the combustion of CH3NO2.
The balanced chemical equation of the reaction is:
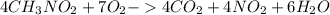
So, from the balanced chemical equation, it is clear that:
4 moles of CH3NO2 --- 7 moles of oxygen gas is required.
then,
for 17.10 moles of CH3NO2 the following number of moles of oxygen is required.
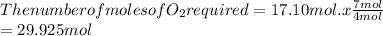
Answer is :
29.9 mol of oxygen gas is required.