Answer:

Step-by-step explanation:
The percent yield is the ratio of the actual yield to the theoretical yield.
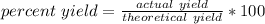
- The actual yield is the amount obtained from performing a chemical reaction. For this problem, it is 14.8 grams.
- The theoretical yield is the potential amount from performing a chemical reaction at maximum performance. For this problem, it is 23.5 grams.
We can substitute the known values into the formula.
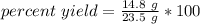
Divide.
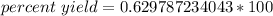
Multiply.
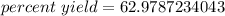
The original measurements for the theoretical and actual yields have 3 significant figures, so our answer must have the same. For the number we calculated, that is the tenths place.
The 7 to the right, in the hundredths place, tells us to round the 9 up to a 0. Since we rounded up to 0, we have to move to the next place to the left and round the 2 up to a 3.
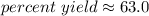
The percent yield is approximately 63.0 percent.