Step-by-step explanation:
The given chemical equation is:
Fe(CN)63-(aq) + Re(s)-> Fe(CN)64-(aq) + ReO4-(aq)
Consider oxidation half reaction and balance it first in acidic conditions:
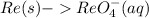
Add water on the left side to balance the O-atoms:
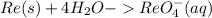
Add protons on the right side to balance H-atoms:
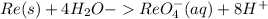
To balance the charge add electrons:
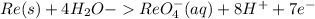 ------------(1)
------------(1)
Reduction half reaction:
Fe(CN)63-(aq) -> Fe(CN)64-(aq)
Add electrons to balance the charge:
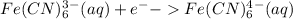 ---------------(2)
---------------(2)
Multiply equation(2) with seven :
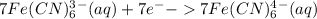 ------(3)
------(3)
Add (1) and (3)
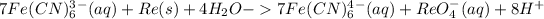
Add 8OH- on both sides:
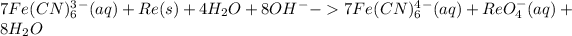
It becomes:
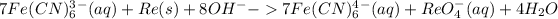
This is the final equation in the basic medium.
Re(s) is oxidised. So it is the reducing agent.
Fe(CN)63- is reduced.It is the oxidising agent.