Answer:
C. The mass of a nucleus is subtracted from the mass of its components.
Step-by-step explanation:
The difference in the mass of a stable nucleus and the combined masses of its constituents (i.e. nucleons) is called “Mass Defect” or “Mass Deficit”. It is denoted by Δm.
Symbolically,
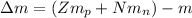
where,
Δm = mass defect
Z = Atomic Number
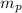 = mass of Proton
= mass of Proton
N = Neutron Number
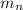 = mass of Neutron
= mass of Neutron
m = mass of Nucleus
Hence, the correct option is:
C. The mass of a nucleus is subtracted from the mass of its components.