Answer:
It increases but less than double
Step-by-step explanation:
As the temperature of a gas increase, the average kinetic energy of the gas increases. The kinetic energy of a gas is the thermal energy that the gas contains.
We know, the kinetic energy of an ideal gas is given by :
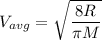
where, R = gas constant
T = absolute temperature
M = molecular mass of the gas
From the above law, we get
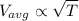
Thus, if we increase the temperature then the average kinetic energy of the ideal gas increases.
In the context, if the temperature of the ideal gas increases from 100°C to 200°C, then
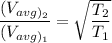
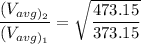
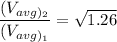
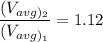
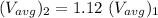
Therefore,
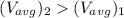
Thus the average kinetic energy of the molecule increases but it increases 1.12 times which is less than the double.
Thus, the answer is " It increases but less that double".