Answer:
1. 26.30 g C.
2. 4.42 g H.
3. 35.04 g O.
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to calculate the required as follows:
1. Here, the only source of carbon is in CO2, and thus, we calculate the grams of carbon from the produced grams of this substance:
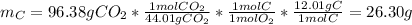
2. Here, the only source of hydrogen is in H2O, and thus, we calculate the grams of hydrogen from the produced grams of this substance:
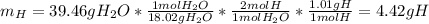
3. Here, we subtract the mass of H and C from the mass of the sample, to obtain the mass of oxygen:
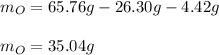
Regards!