Answer:
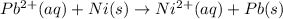
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out firstly necessary for us to write the complete molecular equation as shown below:
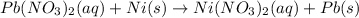
Now, we can separate the nitrates in ions as they are aqueous to obtain:
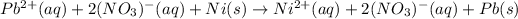
And then, we cancel out the nitrate ions as the spectator ones, for us to obtain the net ionic equation:
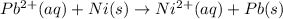
Best regards!