Answer:
Gold
Step-by-step explanation:
We are given that
Mass of sample ,m=385 g
Volume ,V=20mL
We have to find the coin is gold or yellow brass.
We know that
Density=
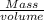
Using the formula
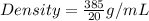
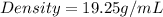
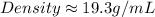
Density of coin=19.3g/mL
Density of gold=19.3g/mL
Hence, the coin is gold.