Answer:
m = 1125.9 g.
Step-by-step explanation:
Hey there!
In this case, according to the given information, it turns out possible for us to solve this problem by using the definition of density as mass divided by volume:
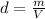
Thus, we solve for the mass in the equation to obtain:
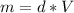
Then, we plug in the values to obtain:
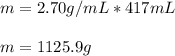
Regards!