Answer:
The wavelength of the incoming photon is 172.8 nm
Step-by-step explanation:
The wavelength of the incoming photon can be calculated with the photoelectric equation:
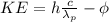 (1)
(1)
Where:
KE: is the kinetic energy of the electron
h: is Planck's constant = 6.62x10⁻³⁴ J.s
c: is the speed of light = 3.00x10⁸ m/s
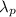 : is the wavelength of the photon =?
: is the wavelength of the photon =?
Φ: is the work function of the surface (Iron) = 4.5 eV
The kinetic energy of the electron is given by:
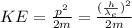 (2)
(2)
Where:
p: is the linear momentum = h/λ
m: is the electron's mass = 9.1x10⁻³¹ kg
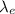 : is the wavelength of the electron = 0.75 nm = 0.75x10⁻⁹ m
: is the wavelength of the electron = 0.75 nm = 0.75x10⁻⁹ m
Hence, the wavelength of the photon is:
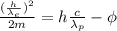
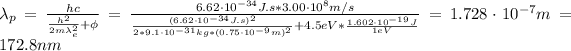
Therefore, the wavelength of the incoming photon is 172.8 nm.
I hope it helps you!