Answer:
Therefore mole ratio is
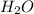 :
:
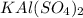 is 12 :1
is 12 :1
Empirical formula is
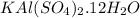 .
.
Step-by-step explanation:
The chemical formula of a hydrate
Moles of anhydrous
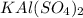
Molar mass of
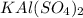 = 258.21 g /mol
= 258.21 g /mol
Mass of anhydrous
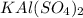 = [mass of aluminum cup + alum after 2nd heating] –[ mass of empty cup]
= [mass of aluminum cup + alum after 2nd heating] –[ mass of empty cup]
= 3.5 g – 2.4 g
= 1.1 g
Moles of
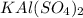 = mass / molar mass
= mass / molar mass
= 1.1 g / 258.21 g per mol
= 0.00426 mol
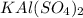
The mole ratio of the H2O to
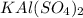
Mole ratio = moles of H2O/ moles of
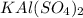
= 0.05 mol H2O / 0.00462 mol
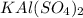
= 11.7
We can round the 11.7 to closest whole number = 12
Therefore mole ratio is
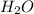 :
:
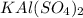 is 12 :1
is 12 :1
Empirical formula =
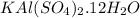
So we have 12 moles of water as the water of hydration in the empirical formula.
Alum hydrate is that the white crystalline solid after heating it'll start melting due to the water of hydration present in it then again solid will remain within the aluminum cup once all the water is given off within the sort of vapors.
b) if the scholar used 2.20 g of the sample but within the calculation, he started with 2.0 g sample then
when the mass of water is calculated using this data the mass of water is going to be higher therefore it gives more moles of water within the hydrate.
So the final answer is going to be artificially high.