Answer:
The resulting pressure is 3 times the initial pressure.
Step-by-step explanation:
The equation of state for ideal gases is described below:
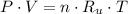 (1)
(1)
Where:
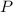 - Pressure.
- Pressure.
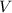 - Volume.
- Volume.
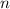 - Molar quantity, in moles.
- Molar quantity, in moles.
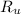 - Ideal gas constant.
- Ideal gas constant.
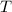 - Temperature.
- Temperature.
Given that ideal gas is compressed isothermally, this is, temperature remains constant, pressure is increased and volume is decreased, then we can simplify (1) into the following relationship:
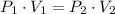 (2)
(2)
If we know that
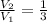 , then the resulting pressure of the system is:
, then the resulting pressure of the system is:
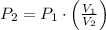
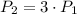
The resulting pressure is 3 times the initial pressure.