Answer:
The molality of solution=12.605 m
Step-by-step explanation:
We are given that
Molar mass of Hydrogen peroxide, M=34 g/mol
Density of solution,
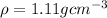
30% Means mass of solute (Hydrogen peroxide)=30 g
Mass of solvent =100-30=70 g
Total mass of solution, m=100 g
Number of moles of solute=
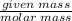
Using the formula
Number of moles of hydrogen peroxide=
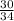
Now, molality of solution
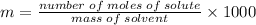
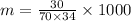
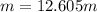
Hence, the molality of solution=12.605 m