Answer:
An unsaturated solution.
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to firstly realize we need to calculate the grams of vanillin in 0.070 moles by using its molar mass of 152.15 g/mol:
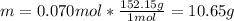
Thus, since the solubility is 10.65 g per 1 L of solution, we can notice 5.00 g will complete dissolve and produce an unsaturated solution.
Best regards!