Answer:
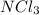
Step-by-step explanation:
An octet rule is a thumb rule in the chemical sciences in which there is a natural tendency for an atom to prefer eight electrons in the valence shell of the atom. When there are less than eight electrons in the atom, they react with other atoms and form more stable compounds.
In the context, nitrogen trichloride,
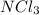 , is an example of molecular compound which obeys the octet rule having a zero formal charges on each atom of the compound.
, is an example of molecular compound which obeys the octet rule having a zero formal charges on each atom of the compound.