Answer:
The mass of silver carbonate precipitated is 5.18 grams.
Step-by-step explanation:
Molarity of the silver nitrate solution = 0.671 M
Volume of the silver nitrate solution = 56.0 mL
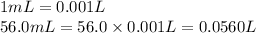
Moles of silver nitrate = n
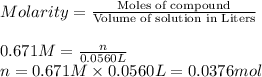
Moles of silver nitrate used = 0.0376 mol
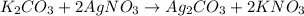
According to the reaction, 2 moles of silver nitrate gives 1 mole of silver carbonate, then 0.0376 moles of silver nitrate:
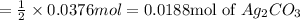
Moles of the silver carbonate formed = 0.0188 mol
Molar mass of silver carbonate = 275.7453 g/mol
Mass of silver carbonate :
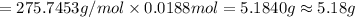
The mass of silver carbonate precipitated is 5.18 grams.