Answer:
1. Combination (Synthesis) reaction
A + B → AB
2. Decomposition reaction
AB → A + B
3. Displacement reaction
A + BC → AB + C
4. Double displacement reaction
AB + CD → AD + BC
5. Combustion reaction
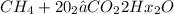
A combination reaction, also known as a synthesis reaction, is a reaction in which two or more substances combine to form a single new substance. Combination reactions can also be called synthesis reactions.
A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances.
A single-replacement reaction is a reaction in which one element replaces a similar element in a compound.
A double-replacement reaction is a reaction in which the positive and negative ions of two ionic compounds exchange places to form two new compounds.
A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion reactions must involve O2 as one reactant. The combustion of hydrogen gas produces water vapor
Explanation: Hope this helps and good luck :))