Answer:
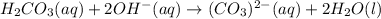
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to set up this net ionic equation, by firstly setting up the complete molecular equation as follows:
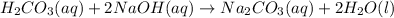
Thus, since carbonic acid is weak it merely ionizes whereas sodium hydroxides ionizes for the 100 % as it is strong; thus, we can write the complete ionic equation:
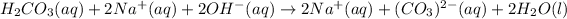
Whereas sodium ions act as the spectator ones to be cancelled out for us to obtain:
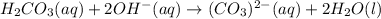
Regards!