Answer:
4.858 g
Step-by-step explanation:
Start with the formula
density =
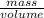
density = 1.98 g/mL
volume = 2.45 mL
mass = ??
rearrange the formula to solve for mass
(density) x (volume) = mass
Add in the substitutes and solve for mass
1.98 g/mL x 2.45 mL = 4.858 g