Answer: There are 0.779 moles of gas were added to the bottle.
Step-by-step explanation:
Given:
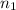 = 0.650 mol,
= 0.650 mol,
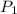 = 730 mm Hg (1 mm Hg = 0.00131579 atm) = 0.96 atm
= 730 mm Hg (1 mm Hg = 0.00131579 atm) = 0.96 atm
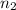 = ?,
= ?,
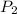 = 1.15 atm
= 1.15 atm
Formula used is as follows.
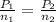
Substitute the values into above formula as follows.
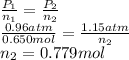
Thus, we can conclude that there are 0.779 moles of gas were added to the bottle.