Answer:
M=0.549M
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to perform this calculation by firstly assuming we have 1 kg of water as the solvent so that we have 0.550 moles of NaCl as well. Moreover, we realize we have 1000 grams of water and the correct mass of the solution can be calculated by converting 0.550 moles of NaCl to grams by using its molar mass:
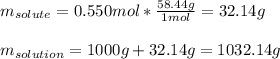
And subsequently, the volume in liters by using the density and the correct conversion factor:
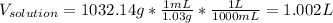
Finally, the molarity will be:
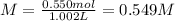
Regards!