Answer: Sugar solution shows no current flow in an electrolytic cell.
Step-by-step explanation:
Electric current is the flow of ions or charged species from one point to another.
A solution that does not contain any ions is not able to conduct electric current.
- Water solution of table salt (NaCl) contains sodium and chlorine ions. So, an electric current can pass through it.
- Molten sodium chloride also contains sodium and chlorine ions. Hence, an electric current can pass through it.
- Hydrochloric acid solution contains
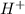 and
and
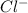 ions. So, an electric current can pass through it.
ions. So, an electric current can pass through it. - Sugar solution does not contain ions as sugar only dissolves in water but do not dissociate into ions. Hence, current cannot flow through sugar solution.
Thus,, we can conclude that sugar solution shows no current flow in an electrolytic cell.