Answer:
The answer is "It takes 1,70 mol of ethanol".
Explanation:
To make acetic acid, we must first write the balanced reaction that occurs of ethanol with oxygen
The response is balanced:
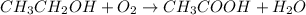
1 mol of ethanol creates 1 mol of According the equilibrium Ethanol moles, therefore, required 1.70 mol of water = 1.70 mol