Answer:
108.6 g
Step-by-step explanation:
- 2NaN₃(s) → 2Na(s) + 3N₂(g)
First we use the PV=nRT formula to calculate the number of nitrogen moles:
- R = 0.082 atm·L·mol⁻¹·K⁻¹
- T = 0 °C ⇒ 0 + 273.2 = 273.2 K
Inputting the data:
- 1.00 atm * 56.0 L = n * 0.082 atm·L·mol⁻¹·K⁻¹ * 273.2 K
Then we convert 2.5 moles of N₂ into moles of NaN₃, using the stoichiometric coefficients of the balanced reaction:
- 2.5 mol N₂ *
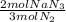 = 1.67 mol NaN₃
= 1.67 mol NaN₃
Finally we convert 1.67 moles of NaN₃ into grams, using its molar mass:
- 1.67 mol * 65 g/mol = 108.6 g