Answer:
highest boiling point.
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out necessary for us to recall the definition of colligative properties, more specifically that of boiling point elevation, defined in terms of the temperature change, van't Hoff's factor, molality of the solution and boiling point elevation constant:
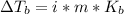
In such a way, since all the given solutions have the same molality and solvent (water), they all have the same m and Kb, for which we focus on the van't Hoff's factor which is 1 for CH2O (nonionizing), 2 for NaCl (Na and Cl ions are released), 2 for HF (H and F ions are released) and 3 for AlCl₃ (one Al and three Cl ions are released).
Therefore, since D. 0.50 mol AICI, in 1.0 kg water has the greatest van't Hoff's factor, we infer it has the highest boiling point.
Regards!