Answer: The electronic configuration of selenium is
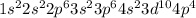
Step-by-step explanation:
The atomic number is defined as the number of protons or number of electrons present in a neutral atom.
Number of electrons will be equal to the atomic number of an atom.
Electronic configuration is defined as the representation of electrons in an atom.
Selenium is the 34th element of the periodic table.
The electronic configuration of Selenium is
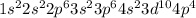
It has 6 electrons that are present in the outermost shell.