Answer:
A. TA = TB/2.
Step-by-step explanation:
Since container A has half as many molecules of the ideal gas in it as container B. Therefore, container A will have half the volume of gas as in container B:
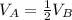
Now, from Charle's Law:
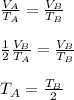
Hence, the correct option is:
A. TA = TB/2.