Answer: A volume of 37.5 mL of 0.300 M NaF would be required to make a 0.0450 M solution of NaF when diluted to 250.0 mL with water.
Step-by-step explanation:
Given:
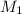 = 0.300 M,
= 0.300 M,
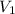 = ?
= ?
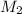 = 0.0450 M,
= 0.0450 M,
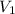 = 250.0 mL
= 250.0 mL
Formula used is as follows.
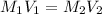
Substitute the values into above formula as follows.
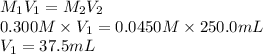
Thus, we can conclude that a volume of 37.5 mL of 0.300 M NaF would be required to make a 0.0450 M solution of NaF when diluted to 250.0 mL with water.