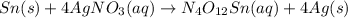Answer: For the given reaction-
- The word equation is Tin + silver nitrate = tin (IV) nitrate + silver
- The skeletal equation is
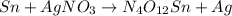
- The balanced chemical equation is
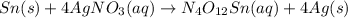
Step-by-step explanation:
- When names of chemicals or species involved in a chemical reaction are represented in the form of an equation then it is known as a word equation.
For example, Tin + silver nitrate = tin (IV) nitrate + silver
The 'equal to' symbol denotes a forward arrow.
- A skeletal equation contains only the chemical formula of species involved in a chemical reaction.
For example,
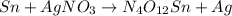
- A chemical equation which contains same number of atoms on both reactant and product side along with states of species is called a balanced chemical equation.