Answer:
21.9% is the experimental percentage of water in the unknown hydrate.
Step-by-step explanation:
The mass of an unknown hydrate = 1.000 g
The mass of hydrate after heating = 0.781 g
The mass of water lost due to heating = x
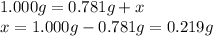
The experimental percentage of water in the unknown hydrate:
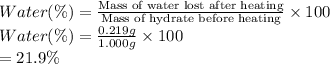
21.9% is the experimental percentage of water in the unknown hydrate.