Answer: The product of the given reaction is
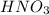 and the solution at equilibrium will be acidic.
and the solution at equilibrium will be acidic.
Step-by-step explanation:
When two or more chemical substances react together then it forms new substances and these new substances are called products.
For example,
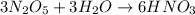
This shows that nitric acid
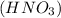 is the product formed and it is an acidic substance.
is the product formed and it is an acidic substance.
Hence, the solution at equilibrium will be acidic in nature.
Thus, we can conclude that the product of the given reaction is
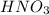 and the solution at equilibrium will be acidic.
and the solution at equilibrium will be acidic.