Answer:
0.350 L
Step-by-step explanation:
- 2Na₂O₂ + 2H₂O → 4NaOH + O₂
First we convert 2.18 g of Na₂O₂ into moles, using its molar mass:
- 2.18 g ÷ 78 g/mol = 0.0279 mol Na₂O₂
Then we convert 0.0279 moles of Na₂O₂ into moles of O₂, using the stoichiometric coefficients of the reaction:
- 0.0279 mol Na₂O₂ *
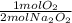 = 0.0140 mol O₂
= 0.0140 mol O₂
Finally we use the PV=nRT formula to calculate the volume of oxygen produced:
- R = 62.36 torr·L·mol⁻¹·K⁻¹
- T = 17.7 °C ⇒ 17.7 + 273.2 = 290.9 K
Inputting the data:
- 726 torr * V = 0.0140 mol * 62.36 torr·L·mol⁻¹·K⁻¹ * 290.9 K