Answer:
For 1: 6.68 g of nitrogen dioxide will contain
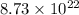 number of molecules
number of molecules
For 2: The given amount of nitrogen dioxide molecules has a mass of 41.31 g.
Step-by-step explanation:
According to the mole concept:
1 mole of a compound contains
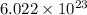 number of molecules
number of molecules
The number of moles is defined as the ratio of the mass of a substance to its molar mass. The equation used is:
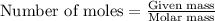 ......(1)
......(1)
We are given:
Mass of nitrogen dioxide = 6.68 g
Molar mass of nitrogen dioxide = 46 g/mol
Putting values in equation 1, we get:
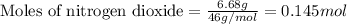
Using above concept:
If 1 mole of a compound contains
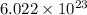 number of molecules
number of molecules
So, 0.145 moles of nitrogen dioxide will contain =
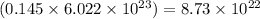 number of molecules
number of molecules
Hence, 6.68 g of nitrogen dioxide will contain
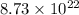 number of molecules
number of molecules
We are given:
Molecules of nitrogen dioxide =
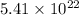 molecules
molecules
Using the above concept:
If
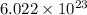 number of molecules are present in 1 mole of a compound
number of molecules are present in 1 mole of a compound
So,
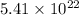 number of molecules will be present in =
number of molecules will be present in =
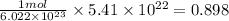 moles of nitrogen dioxide
moles of nitrogen dioxide
We know, molar mass of nitrogen dioxide = 46 g/mol
Using equation 1:
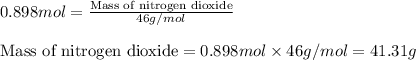
Hence, the given amount of nitrogen dioxide molecules has a mass of 41.31 g.