Answer:
65.2%
Step-by-step explanation:
It is given that :
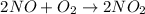
Therefore, 60 g of nitrogen oxide will produce 92 g of nitrogen dioxide
or 60 kg of nitrogen oxide will produce 92 kg of nitrogen dioxide
or 1500 kg of nitrogen oxide will produce
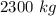 of nitrogen dioxide
of nitrogen dioxide
Therefore the percentage yield = (Actual yield / Expected yield) x 100
percentage yield =
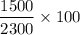
Percentage yield = 65.217 %