Answer:
0.605 g
Step-by-step explanation:
- MnO₂(s) + 4HCl(aq) ⟶ MnCl₂(aq) + 2H₂O(l) + Cl₂(g)
First we calculate how many Cl₂ moles need to be produced, using the PV=nRT formula:
- P = 745 Torr ⇒ 745 / 760 = 0.980 atm
- V = 185 mL ⇒ 185 / 1000 = 0.185 L
- R = 0.082 atm·L·mol⁻¹·K⁻¹
- T = 25 °C ⇒ 25 + 273.16 = 298.16 K
Inputting the data:
- 0.980 atm * 0.185 L = n * 0.082 atm·L·mol⁻¹·K⁻¹ * 298.16 K
Then we convert 0.00696 moles of Cl₂ to MnO₂ moles:
- 0.00696 mol Cl₂ *
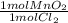 = 0.00696 mol MnO₂
= 0.00696 mol MnO₂
Finally we convert 0.00696 moles of MnO₂ to grams, using its molar mass:
- 0.00696 mol MnO₂ * 86.94 g/mol = 0.605 g