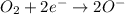Answer: The balanced half-equations for silver + oxygen= silver oxide are:
Oxidation-half reaction:
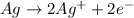
Reduction-half reaction:
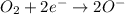
Step-by-step explanation:
The word equation is as follows.
silver + oxygen = silver oxide
In terms of chemical formulas this equation can be written as follows.

The removal on electron(s) from an atom, ion or molecule in a chemical reaction is called oxidation.
The gain of electron(s) by an atom, ion or molecule in a chemical reaction is called reduction.
Hence, half-reaction equations for the given reaction is as follows.
Oxidation-half reaction:
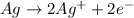
Reduction-half reaction:
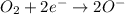
As the number of atoms participating in the reaction are equal. Hence, the half-equations are balanced.
Thus, we can conclude that the balanced half-equations for silver + oxygen = silver oxide are:
Oxidation-half reaction:
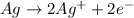
Reduction-half reaction: