Answer:
the percent dissociation is 0.69 %
Step-by-step explanation:
Given the data in the question;
benzoic acid C₆H₅CO₂H
C₆H₅COOH
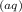 ⇔ C₆H₅COO
⇔ C₆H₅COO
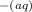 + H
+ H
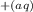
Ka = [C₆H₅COO- ][ H+ ] / [ C₆H₅COOH ] = 6.28 × 10⁻⁵
given that it dissociated in a 1.3 M aqueous solution.
so Initial concentration is;
[ C₆H₅COOH ] = 1.3
[C₆H₅COO- ] = 0
[ H+ ] = 0
Change in concentration
[ C₆H₅COOH ] = -x
[C₆H₅COO- ] = +x
[ H+ ] = +x
Concentration equilibrium
[ C₆H₅COOH ] = 1.3 - x
[C₆H₅COO- ] = +x
[ H+ ] = +x
Hence,
x² / ( 1.3 - x ) = 6.28 × 10⁻⁵
6.28 × 10⁻⁵( 1.3 - x ) = x²
8.164 × 10⁻⁵ - 6.28 × 10⁻⁵x = x²
x² + 6.28 × 10⁻⁵x - 8.164 × 10⁻⁵ = 0
solve for x
ax² + bx - c = 0
x = [ -b ± √( b² - 4ac ) ] / [ 2a ]
we substitute
x = [ -6.28 × 10⁻⁵ ± √( (6.28 × 10⁻⁵)² - (4 × 1 × -8.164 × 10⁻⁵ ) ) ] / [ 2 × 1 ]
x = [ -6.28 × 10⁻⁵ ± 0.01807 ] / [ 2]
x = [ -6.28 × 10⁻⁵ - 0.01807 ] / [ 2] or [ -6.28 × 10⁻⁵ + 0.01807 ] / [ 2]
x = -0.0090664 or 0.0090036
so x = 0.0090036
hence
[ H+ ] = +x = 0.0090036 M
[C₆H₅COO- ] = +x = 0.0090036 M
Initial concentration of [ C₆H₅COOH ] = 1.3 M
concentration of C₆H₅COOH dissociated = 0.0090036 M
percent dissociation of C₆H₅COOH will be;
⇒ ( 0.0090036 M / 1.3 M ) × 100 = 0.69 %
Therefore, the percent dissociation is 0.69 %