Answer:
The produced salt is calcium chloride, CaCl₂, whose cation, Ca²⁺, is the conjugate acid of the base and the anion, Cl⁻ the conjugate base of the acid.
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out firstly necessary for us to set up the chemical equation between calcium hydroxide and hydrochloric acid:
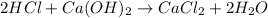
It means that the produced salt is calcium chloride, CaCl₂, whose cation, Ca²⁺, is the conjugate acid of the base and the anion, Cl⁻ the conjugate base of the acid.
Regard"