Answer:
A sample of calcium fluoride was decomposed into the constituent elements. Write a balanced chemical equation for the decomposition reaction. If the sample produced 294 mg of calcium, how many g of fluorine was formed
Step-by-step explanation:
The balanced chemical equation for the decomposition of calcium fluoride is shown below:
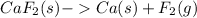
The sample produced 294 g of calcium then, how many grams of fluorine is formed?
From the balanced chemical equation,
1 mol of CaF2 forms 1mol of calcium and 1 mol of fluorine.
That is:
40g of calcium and 38.0 g of fluorine are formed.
then,
If 294 g of calcium is formed then how many grams of fluorine is formed?
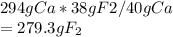
Hence, 279.3 g of fluorine will be formed.