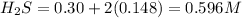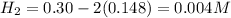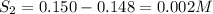Answer:
Equilibrium concentrations of the gases are
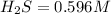
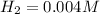
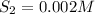
Step-by-step explanation:
We are given that for the equilibrium
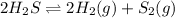
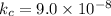
Temperature,
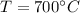
Initial concentration of
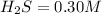
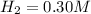
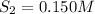
We have to find the equilibrium concentration of gases.
After certain time
2x number of moles of reactant reduced and form product
Concentration of
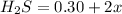
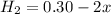
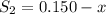
At equilibrium
Equilibrium constant
![K_c=(product)/(Reactant)=([H_2]^2[S_2])/([H_2S]^2)](https://img.qammunity.org/2022/formulas/chemistry/high-school/8ptyb0waea93tw4gaga98t13d1kxwoxvr7.png)
Substitute the values
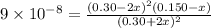
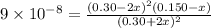
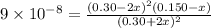
By solving we get
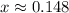
Now, equilibrium concentration of gases