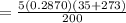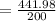Answer:
The right answer is "2.2099 m³".
Step-by-step explanation:
Given:
Mass,
m = 5 kg
Temperature,
T = 35℃
or,
= 35 + 273
Pressure,
P = 200 kPa
Gas constant,
R = 0.2870 kj/kgK
By using the ideal gas equation,
The volume will be:
⇒
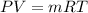
or,
⇒
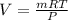
By substituting the values, we get