Answer:
Final pressure, P2 = 1088.89 atm
Step-by-step explanation:
Given the following data;
- Initial volume, V1 = 725 cm³
- Initial temperature, T1 = 30°C
- Initial pressure, P1 = 1.19 atm
- Final volume, V2 = 1.12 cm³
- Final temperature, T2 = 43°C
To find the final pressure (P2), we would use the combined gas law.
Mathematically, the combined gas law is given by the formula;
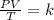
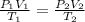
Substituting into the formula, we have;
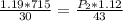
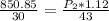
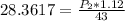
Cross-multiplying, we have;
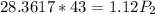

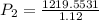
Final pressure, P2 = 1088.89 atm