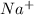Answer: Most likely ion symbol for given element is as follows.
- Scandium -
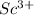
- Fluorine -
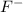
- Sulfur -
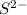
- Calcium -
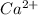
- Sodium -
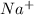
Step-by-step explanation:
When an element loses an electron in order to acquire stability then it is called a cation. Metals always form cations and cations have a positive charge.
For example, scandium is a metal and
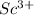 is a cation.
is a cation.
Atomic number of calcium is 20 (2, 8, 8, 2). So, it loses 2 electrons to acquire stability and hence forms
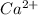 ion.
ion.
Atomic number of sodium is 11 (2, 8, 1). So, it loses 1 electron to acquire stability and hence forms
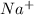 ion.
ion.
When an element gains an electron in order to complete its octet then it is called an anion. Non-metals always form anions and anions have a negative charge.
For example, atomic number of fluorine is 9 (2, 7). So, it will gain one electron to acquire stability and hence forms
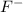 ion.
ion.
Atomic number of sulfur is 16 (2, 8, 6). So, it will gain 2 electrons to acquire stability and hence forms
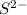 ion.
ion.
Thus, we can conclude that most likely ion symbol for each given element is as follows.
- Scandium -
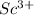
- Fluorine -
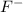
- Sulfur -
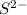
- Calcium -
- Sodium -