The question is as follows: How many oxygen molecules are produced by the decomposition of 28.5 g of H2O2 (molecular mass = 34.0g / mol) according to the equation
2H2O2 (l) → 2H2O (l) + O2 (g)
Answer: There are
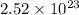 molecules are produced by the decomposition of 28.5 g of
molecules are produced by the decomposition of 28.5 g of
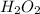 according to the equation
according to the equation
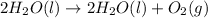 .
.
Step-by-step explanation:
Given: Mass of
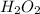 = 28.5 g
= 28.5 g
As moles is the mass of a substance divided by its molar mass. Hence, moles of
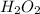 is calculated as follow.
is calculated as follow.
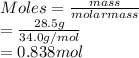
According to the given equation, 2 moles of
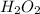 gives 1 mole of
gives 1 mole of
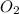 . So, moles of
. So, moles of
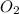 produced by 0.838 moles of
produced by 0.838 moles of
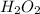 will be calculated as follows.
will be calculated as follows.
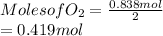
This means that moles of
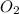 produced is 0.419 mol.
produced is 0.419 mol.
As per the mole concept, 1 mole of every substance has
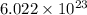 molecules.
molecules.
So, molecules of
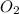 present in 0.419 mole are as follows.
present in 0.419 mole are as follows.
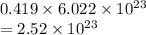
Thus, we can conclude that there are
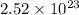 molecules are produced by the decomposition of 28.5 g of
molecules are produced by the decomposition of 28.5 g of
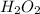 according to the equation
according to the equation
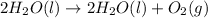 .
.