Answer:
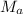 = 0.104 m
= 0.104 m
Step-by-step explanation:
This expression is used to determine either the mass or volume of an acid or a base used during titration process.
So that;
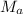
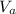 =
=
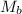
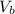
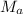 is the mass of the acid
is the mass of the acid
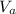 is the volume of the acid
is the volume of the acid
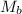 is the mass of the base
is the mass of the base
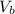 is the volume of the base
is the volume of the base
Given that:
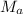 x 5.0 m l = 5.2 ml x 0.10 m
x 5.0 m l = 5.2 ml x 0.10 m
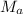 x 5.0 = 0.52 m
x 5.0 = 0.52 m
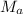 =
=
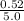
= 0.104
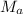 = 0.104 m
= 0.104 m
The mass of the acid used is 0.104 m.